This guide provides general information that you may find useful before starting and while undergoing radiation therapy (RT) for prostate cancer. We hope it helps you understand what to expect from, and how to deal with the various aspects of the treatment. This guide is not meant to be all-inclusive but rather to aid you in having informed discussions with your health care providers. They will be available to answer questions throughout your treatment.
Radiation Therapy for Prostate Cancer
What is radiation therapy?
Radiation therapy uses ionizing radiation in the form of photons or protons to kill cancer cells by damaging their DNA. Cancer cells with damaged DNA cannot divide or multiply and will eventually die. The overarching goal of radiation therapy is to deliver a therapeutic dose of ionizing radiation to the tumor while minimizing damage to surrounding healthy normal tissues. There are many methods for achieving this goal, as detailed below.
Why choose radiation therapy?
RT is an important method of treating prostate cancer. The rationale, risks and benefits of RT depend on the stage and grade of the cancer, and the radiation method employed.
For patients with localized (confined to the prostate), locally advanced (extension through capsule or in seminal vesicles), or regionally advanced prostate cancer (spread to adjacent pelvic lymph nodes only), RT is used with intent to cure and is an alternative to surgery. For those with signs of high-risk prostate cancer who undergo radical prostatectomy, RT may be used as an adjuvant or salvage therapy for a higher likelihood of cure than from surgery alone. For those with metastatic disease, RT may be used to improve survival, delay disease progression, or palliate symptoms caused by tumors. It may be given before, during or after hormone therapy (HT).
You should discuss all treatment options with your health care providers. For additional information on possible alternative therapies at UCSF, see localized prostate cancer.
Types of radiation therapy
There are two broad categories of radiation therapy: external beam radiation therapy (EBRT) and brachytherapy. They work as follows:
- In EBRT, an external source of radiation beams photons (X-rays or gamma rays) or particles (protons or heavy ions) into the tumor. In the modern era of radiation oncology, the most common external radiation source is a megavoltage linear accelerator (linac) equipped with devices that allow precise shaping of X-ray photons and accurate tumor targeting.
- In brachytherapy, radioactive sources are implanted in the tumor, either temporarily or permanently.
Prostate cancer may be treated with EBRT alone, brachytherapy alone, or a combination of the two.
Advances in EBRT
Radiation therapy has advanced significantly over the past half century.
In its early days, EBRT was planned by drawing the radiation target on X-ray films, thus guided by only two-dimensional information, typically bony anatomy. With the advent of computers and computed tomography (CT) scans in the 1980s, radiation oncologists began using three-dimensional soft tissue anatomic information to generate 3D conformal radiation therapy (3D-CRT) plans. These made it possible to reduce radiation exposure to healthy tissues, thereby permitting delivery of higher radiation doses to the tumor. Further advances in the 1990s enabled computer-optimized radiation planning with a technique called intensity-modulated radiation therapy (IMRT), in which the radiation beam is divided into "beamlets" that are individually adjusted to conform radiation doses to the tumor's shape. In the late 1990s and 2000s, a sophisticated form of IMRT called volumetric modulated arc therapy (VMAT) was invented. VMAT involves continuous modulation of the radiation beam as the linac gantry – the frame holding the radiation source – rotates in an arc. VMAT and IMRT are now the techniques most often used in planning and delivering external photon beam treatment.
Comparable advances have been made in proton beam therapy over time, with older 3D conformal and newer pencil-beam scanning technologies. Whereas photons pass through the body entirely, protons enter the body but stop within the targeted tissue or organ, thereby eliminating an "exit dose" of radiation to organs on the other side of the target. In theory, this method would reduce radiation exposure to healthy tissues, but studies have yet to prove proton therapy is more effective than photon therapy in curing prostate cancer or improving quality of life.
In addition to advances in radiation planning techniques, tumor targeting has improved through image-guided radiation therapy (IGRT). This is important for treating prostate cancer because the prostate is not a fixed target – its position changes with patient movement, bladder filling and emptying, and the passage of gas or stool. IGRT permits positional adjustments during radiation therapy to compensate for these changes. Examples of IGRT include orthogonal X-ray imaging of implanted gold seed fiducial (reference) markers, low-dose cone-beam CT scans, and real-time intra-fraction target tracking during radiation delivery.
At UCSF, to treat prostate cancer with EBRT, we use either a gantry-based linac (such as the Varian TrueBeam), equipped with the latest IGRT technology, or the Accuray CyberKnife, a linac mounted on a robotic arm with intra-fraction target tracking capability. These technologies enable us to provide state-of-the-art care.
EBRT fractionation
EBRT is typically delivered over multiple treatment sessions called fractions because each treatment is a fraction of the total prescribed dose. Each fraction is the same amount – that is, the total prescribed radiation dose is divided into equal daily doses. Treatments are delivered on weekdays only.
This strategy allows your healthy noncancerous tissue to repair itself between treatments. Cancer cells are not as efficient at DNA damage repair as normal tissues. Fractionation also allows different populations of cancer cells to be irradiated.
The radiation dose delivered with each fraction is measured in gray, a scientific unit of energy per unit mass (1 gray, or Gy, is equal to 1 joule per kilogram).
Treatment can be fractionated in a number of ways:
- Conventional fractionation refers to 1.8 to 2.0 Gy per fraction. A course of conventionally fractionated radiotherapy for curative treatment of prostate cancer typically involves 35 to 45 fractions total, which takes about eight to nine weeks to complete.
- Hypofractionation refers to radiation therapy delivered with a higher dose per fraction over a shorter time. Because each fraction is a higher percentage of the prescribed radiation dose, accuracy in targeting the prostate is essential.
- Moderate hypofractionation refers to 2.5 to 3.1 Gy per fraction, typically delivered in 20 to 28 fractions over four to six weeks. Several studies show moderate hypofractionation is as effective in achieving a cure for localized prostate cancer as conventional fractionation — and has the added benefits of being more convenient and cost effective.
- Ultra-hypofractionation refers to treatment with more than 6 Gy delivered per fraction and typically three to seven fractions total.
- Stereotactic body radiation therapy (SBRT) or stereotactic ablative body radiotherapy (SABR) delivers ultra-hypofractionation in five or fewer fractions using modern image-guidance techniques for targeting accuracy. At UCSF, prostate SBRT is delivered on either the Varian TrueBeam or the Accuray CyberKnife.
Brachytherapy
In brachytherapy, small radioactive seeds (each the size of a grain of rice) are implanted directly into the tumor to maximize the radiation dose to the cancer cells and minimizing radiation exposure for normal tissues. The entire prostate gland also is implanted with radioactive seeds, because the whole gland is considered to be at risk, even if the biopsy or imaging suggests the tumor is confined to one part of it. The exception to this rule is for patients receiving brachytherapy due to focal recurrence in the prostate after prior radiation. In that specific scenario, there may be a decision to implant only the area of recurrence.
In patients with low-risk or favorable intermediate-risk prostate cancer, brachytherapy may be used alone. In patients with unfavorable intermediate-risk or high-risk prostate cancer, brachytherapy may be used to boost the radiation dose to the prostate in combination with EBRT targeting the pelvic lymph nodes or seminal vesicles. The brachytherapy boost has been shown to provide better disease control than EBRT alone, though it can increase the risk of urinary side effects.
The two types of prostate brachytherapy are low dose rate and high dose rate.
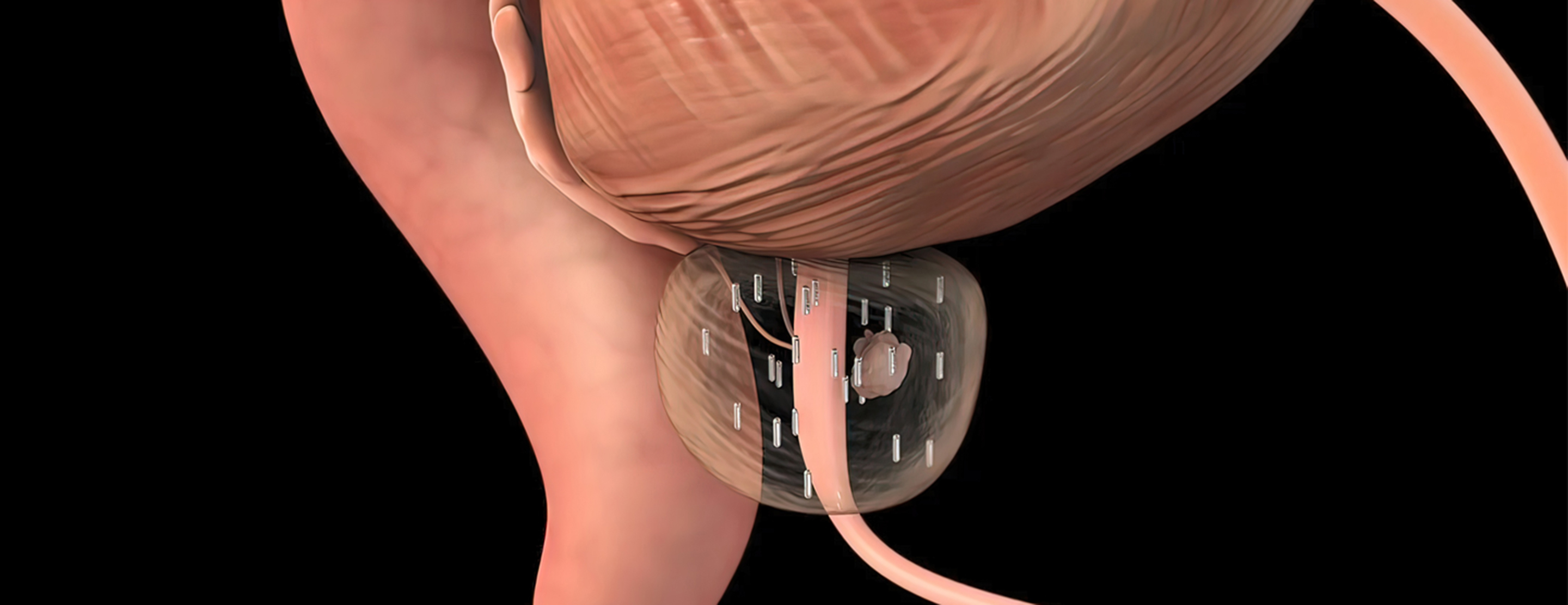
In low dose rate (LDR, or permanent seed) brachytherapy, multiple small radioactive seeds are permanently placed in the prostate. They are implanted through the perineum (area between scrotum and anus), using thin hollow needles guided by transrectal ultrasound. The procedure takes 45 to 60 minutes. Depending on the radioactive isotope used, the seeds deliver radiation to the prostate over weeks to months, eventually becoming inert. They stay in place for life. Careful brachytherapy planning and seed placement result in a dose designed to destroy the cancer cells.
High dose rate (HDR, or temporary seed) brachytherapy involves the temporary placement of 14 to 20 catheters (hollow needles) through the perineum into the prostate. After appropriate catheter placement, a highly radioactive seed mounted on a wire and controlled by a computerized robot called an afterloader is inserted into one catheter at a time. The afterloader systematically moves each seed through a catheter to safely deliver a very high dose to the prostate. The radiation dose can be precisely shaped by controlling how long and exactly where the seed is placed. As in LDR brachytherapy, the entire prostate is targeted, except in cases of salvage brachytherapy (repeated radiation). Unlike LDR brachytherapy, no radioactive material is left behind. HDR is the type of brachytherapy we provide most often at UCSF.
The feasibility of prostate brachytherapy depends on many factors, including the cancer's stage; baseline urinary symptoms; prostate size and anatomy; and prior surgical procedures, such as transurethral resection of the prostate. In patients with a significantly enlarged prostate, gland reduction using 5-alpha-reductase inhibitors (such as finasteride or dutasteride) or hormone therapy may be recommended prior to administering brachytherapy.
At UCSF, we usually provide general, spinal or epidural anesthesia for pain control before performing prostate brachytherapy. The type used depends on the brachytherapy needs and occasionally, patient related factors (for example, prior history of extensive low back surgeries makes it difficult to do spinal or epidural). Learn more about brachytherapy.
Preparing for EBRT
Patients preparing for radiation therapy will have one or two radiation planning appointments before starting treatment. At the first appointment (which may be omitted in some circumstances), tiny nonradioactive gold seeds may be implanted in the prostate or prostate bed (structure on which the prostate rests); these are markers that help the radiation therapist position your body accurately during treatment. The gold seeds are placed using transrectal ultrasound (TRUS) guidance. The procedure is similar to a prostate biopsy, but patients usually find it much less uncomfortable because it's done with a thinner needle and requires fewer needle pokes.
When the seeds are placed, your radiation oncologist may offer you the option of also having a rectal hydrogel spacer placed. The spacer's purpose is to create distance between the prostate and the rectum, buffering the rectum from radiation exposure. Placement is performed under local anesthetic with TRUS-guided needle insertion through the perineum. Hydrogel is injected into the space between the rectum and the prostate as a liquid that solidifies over a few minutes. The hydrogel spacer remains intact for approximately three months and then the material is gradually absorbed by the body and excreted in urine.
The spacer's major potential benefit is a reduction in low-grade radiation-related side effects. Potential risks include temporary pelvic discomfort or pain after placement or, in rare cases, an infection, such as a rectal abscess or a rectal wall perforation requiring antibiotics or surgery. There is controversy within the radiation oncology community as to whether the benefits of rectal hydrogel spacers outweigh the risks. You and your radiation oncologist and urologist should discuss whether this option is right for you.
At the second appointment, you will have a "simulation," a radiation planning session in which a scan of the pelvis is obtained in the treatment position. You may be asked to use an enema prior to the simulation. In addition, you may be asked to drink 20 to 24 ounces of fluids 30 minutes to an hour before the scan so that your bladder is full. Alternatively, you may be asked to empty both rectum and bladder immediately prior to the scan. Make sure you understand your doctor's specified preparation instructions before this appointment.
In most cases, the simulation scan is done with computed tomography (CT), but sometimes a scan with magnetic resonance imaging (MRI) is obtained as well. During the appointment, a series of ink marks may be drawn on your skin as guidance for positioning your body for radiation treatment. These marks may be temporary, or you may have three permanent pinhead-size black dots tattooed – one on the left hip, one on the right hip, and one in the area between belly button and pubic bone.
After the simulation, your radiation oncologist and dosimetrist (a specialist in calculating radiation therapy doses) use a sophisticated computer program to customize a radiation treatment plan to your anatomy. This treatment planning process optimizes the radiation beam arrangements, shape and energy of each beam, and dose delivered, ensuring that the prescribed radiation dose gets to the target and that surrounding normal tissue – including the bladder, bowel and rectum – is protected. After your radiation oncologist approves your plan, your treatment begins.
What to expect during EBRT
The first day of treatment may be longer than other sessions because additional imaging is performed that day as part of quality assurance. In most cases, you'll be scheduled for daily treatments, Monday through Friday. SBRT may be scheduled every other day, instead of daily. You'll be assigned a treatment time slot, typically but not necessarily at the same time each day. A specifically trained radiation therapist will administer your treatments.
During treatment, you'll be asked to lie motionless on the treatment table. The area being treated will be exposed (you may choose to wear a hospital gown) and positioned beneath the linac. To deliver the radiation, the head of the machine will move around your body to predetermined angles. On a gantry-based linac, such as a Varian TrueBeam, each treatment takes from five to 20 minutes. On the CyberKnife, each treatment is typically 30 to 60 minutes. Expect to be in the radiation oncology clinic approximately 30 to 60 minutes each day if the TrueBeam is used or 60 to 90 minutes with the CyberKnife.
Please note that many people will be involved in your treatment – your doctor, residents, nurses, therapists, dosimetrists – but your radiation oncologist will supervise your treatment and ensure all steps are being performed accurately.
On occasion, there may be a technical issue with one of the machines that deliver EBRT and treatment cannot be delivered at your scheduled appointment. If any aspect of the machine's normal function is irregular, a safety feature prevents the machine from operating. If this happens, you will be treated on another machine, wait until the problem is resolved, or skip treatment that day. We will ask you to skip a treatment only when the machine requires a longer-than-usual evaluation and repair time. If you miss a treatment, it will be made up, so you receive the total radiation dose prescribed by your doctor.
Side effects during treatment
Undergoing EBRT is similar to having routine X-rays and no more uncomfortable. Radiation cannot be seen, smelled, or felt. Patients are usually able to continue with their normal daily activities during treatment. But many do experience side effects. If you're having conventional radiation treatments, side effects generally don't appear until the second or third week. They may show up earlier if you're having prostate SBRT, LDR, or HDR brachytherapy.
Because radiation therapy is a local treatment, any side effects will primarily involve the area where the radiation is directed. With prostate cancer, patients may experience some or all of the following:
- Increased urination frequency
- Urinary urgency with rare leakage
- Weak urinary stream
- Difficulty starting urination
- Mild burning or tingling during urination
- Loose stools or (less often) diarrhea
- Softer and smaller bowel movements
- Increased frequency of bowel movements
- Worsening of existing hemorrhoids or (rarely) rectal irritation with occasional scant blood
- Generalized fatigue of varying severity
Despite the fatigue, patients receiving radiation therapy usually find they can function and work as usual. Many studies show that regular exercise during the course of radiation therapy can ease side effects, including fatigue.
If needed, you may be prescribed medications for symptom relief. This could be an antidiarrheal medication (such as Imodium AD or Lomotil) or a medication to decrease urination frequency (such as Flomax or Uroxatral). You may also be advised to adjust your dietary habits or take a daily fiber supplement, such as psyllium (Metamucil). Most of these symptoms will go away a few weeks after your radiation treatment ends. The time for full recovery depends on the individual patient and any degree of urinary or bowel issues the patient had before beginning treatment.
As part of your treatment planning, you'll be asked to fill out questionnaires about your urinary, bowel and sexual health. Be sure to discuss the nature and severity of current symptoms related to these functions in detail with your health care provider, as they may affect your treatment course.
Because radiation therapy irradiates some healthy tissues in addition to the prostate, there's a very small risk of a radiation-induced secondary malignancy occurring within the pelvis more than five years after your treatment; when this happens, it's most often in the bladder or rectum and is very rare. The risk of secondary malignancies is most relevant for younger patients.
What to expect after treatment
Most side effects lessen significantly in the first 3-4 weeks but you may experience urinary and bowel side effects for several weeks after you complete your EBRT treatment. Full recovery however takes three to six months and some patients may never recover to their baseline urinary and bowel function and may need to continue taking medications prescribed during treatment. Rare patients report continued but lessening fatigue for several weeks or months after treatment ends. The better the urinary and bowel functions are prior to radiation, the more likely full recovery occurs.
After both LDR and HDR brachytherapy, you may experience the following:
- burning with urination
- increased urination frequency (daytime and nighttime)
- slow or weak urinary stream
- incomplete emptying of the bladder
- a brief period of blood in urine (usually in the immediate post-procedure period)
- perineal pain or soreness
- scrotal bruising or swelling
- blood spotting from the perineum
- nausea and headache (from spinal or epidural anesthesia)
- fatigue
Most patients are able to keep up with their normal daily activities, although it's recommended that you avoid heavy lifting and strenuous physical activity for two to three days after the implant. In a small number of patients (less than 5%), swelling of the prostate obstructs urinary outflow from the bladder. If this happens, you may go home from the brachytherapy procedure with a urinary catheter (Foley catheter) in place and be prescribed a medication to reduce the inflammation. In the rare case that a urinary obstruction occurs after discharge from the hospital, you should go to the nearest emergency room so that a Foley catheter can be placed. The catheter is usually removed within a few days. Rectal symptoms are uncommon after brachytherapy. There may be some rectal discomfort, but rectal bleeding is rare.
Radiation safety is a common concern for patients undergoing radiation treatment, and you'll receive detailed written instructions on this topic before going home. If you're having EBRT or HDR brachytherapy, there are no radiation safety issues for your loved ones. When daily treatment is complete, no radiation remains in the body, so you pose no danger to others and needn't isolate yourself from family and friends.
If you're being treated with LDR (permanent seed) brachytherapy, your body tissues absorb most of the radiation. However, for the first month or two after the seeds are implanted, you should take precautions around pregnant women and young children. Maintain a distance of at least six feet from any pregnant women in your company for a prolonged period. In addition, you shouldn't let young children or pets rest in your lap for prolonged periods. Sexual intercourse may be resumed any time after the implant, but you should wear a condom for at least the first two weeks after the procedure and at least the first three times you ejaculate, due to a low risk of radioactive material in the ejaculate.
Sexual effects
RT can have many effects on your sexual and reproduction function, some more lasting than others. The following may be affected:
- Erectile function. Some patients may experience erectile dysfunction (ED) or impotence after radiation therapy. The likelihood of impaired function is influenced by patient age and baseline sexual function (the primary risk factors), use and duration of hormone therapy (HT), smoking history, other medical conditions (such as high blood pressure or diabetes), and the medicines used for these. For some patients, erectile function declines gradually over the five years following radiation treatment. Patients may develop some degree of ED after brachytherapy, but it's less likely than after other forms of radiation treatment. Most patients who aren't taking nitrate-containing medications are able to use any of the ED drugs on the market. These medications have variable success in improving erectile quality.
- Orgasm. Most patients can achieve orgasms after RT, but some experience a change in their orgasms. Most patients who didn't have their prostate removed experience a change in their ejaculate (thicker or less watery) and a decrease in quantity (or absence) of ejaculate after radiation treatment. This is usually more common with EBRT than with brachytherapy. Following brachytherapy, ejaculate may be discolored (dark brown or black); this is due to residual blood from the procedure. It is harmless and poses no risk to the patient or his sexual partners. The ejaculate will become clearer over time.
- Sperm production. Germinal cells in the testicles produce sperm. During prostate radiation, low levels of radiation scattered from the treatment beam can reach the testicles and decrease sperm production. The reduction in sperm count is usually temporary (lasting months to years). It is possible, however, that your sperm count may be permanently reduced, leading to sterility. If you're considering fathering children in the future, you may want to seek medical advice regarding your fertility and consider sperm banking prior to starting treatment.
- Testosterone production. Most patients experience no change in testosterone levels from prostate radiation therapy alone. Depending on the technique used, the treatment may cause a modest, temporary decline in testosterone levels due to the testicles absorbing some "scatter radiation" (small amounts of radiation that scatter from the targeted beam when it hits body tissues). Radiation alone is highly unlikely to result in permanent alterations in testosterone levels.
- Effects of hormone therapy. Some patients with high risk or regionally advanced disease may benefit from hormone therapy (ADT) in conjunction with their radiation therapy. ADT can drastically reduce testosterone production during treatment. In some cases—including patients who have low baseline testosterone, are elderly or who receive ADT for more than 12 months — the reduction in testosterone production may be permanent. Extended ADT also can cause the penis to be shorter and less flexible. Patients may experience a loss of erectile function during ADT therapy and are encouraged to talk about this with their doctor. Those who expect to be on hormone therapy for 1-3 years, and want to take steps to preserve their sexual function can ask their doctor for ED treatments such as medications, penis pumps or penile injections.
Managing side effects
Mild fatigue may occur during a course of EBRT. If this happens, daily exercise may help increase energy levels. Most patients who work full-time are able to continue doing so during treatment, but you may wish to consider decreasing your hours or taking a leave. In either scenario, try to remain physically active and eat a well-balanced diet. UCSF cancer patients can receive free nutrition counseling with their doctor's referral. If your fatigue becomes severe, contact your doctor.
Diarrhea, flatulence, or painful defecation may occur after the second or third week of conventionally fractionated radiation therapy or earlier with hypofractionated schedules. For most patients, these symptoms don't require treatment and will go away after radiation therapy is complete. During radiation treatment, modifying your diet can reduce the frequency and severity of diarrhea. Avoid eating fried, greasy or highly spiced foods. Cut down temporarily on foods high in insoluble fiber, such as lettuce and cauliflower, and increase low-fiber and soluble-fiber foods, such as very ripe bananas, mashed potatoes, applesauce, white rice, and canned or cooked fruits and vegetables. Some patients may find it helpful to take a daily soluble fiber supplement, such as psyllium (Metamucil).
Maintain your intake of lean proteins, such as turkey, chicken and fish, and increase your fluid intake to avoid dehydration. Using moist toilet paper or baby wipes and taking sitz baths may help relieve rectal irritation. Your doctor may recommend antidiarrheal medications. Contact your health care provider if you see blood in your stool, if diarrhea worsens, or if you become light-headed or dizzy.
Frequent urination, burning with urination and difficulty urinating are the most common complaints of patients receiving radiation therapy for prostate cancer. Occasionally, the urinary stream will weaken. Generally, these symptoms are not severe and are managed with medications that help the bladder function better or that eliminate burning. Rarely, your health care provider may order a urine test. These symptoms will go away after the end of treatment. Contact your provider if you see blood in your urine or if you are unable to urinate, which can be an emergency.
Swelling, bruising or tenderness of the scrotum may occur after brachytherapy. Swelling and tenderness usually go away within three to five days. Bruising takes longer. Oral anti-inflammatory medications, such as ibuprofen, are generally sufficient for pain relief. Avoid hot tubs and whirlpool baths for at least two or three days after the procedure, and don't ride a bicycle until the tenderness has resolved.
Skin irritation isn't a common side effect, but if it occurs, don't rub or scratch the area. Avoid constrictive clothing, and don't use lotions containing alcohol. Your doctor can recommend a skin care regimen and topical creams to relieve the symptoms.
Post-treatment follow-up
Following EBRT, some patients may have an appointment to make sure that treatment-related side effects are diminishing or have resolved. The frequency of follow-up appointments will be determined by your risk of cancer recurrence. In general, PSA blood tests are performed every three to six months during the first two to three years after completing treatment, then every six months until year five, and then annually. During your follow-up evaluations, your doctor may make changes to this schedule depending on your PSA results.
Patients receiving LDR brachytherapy will have an appointment for a CT scan of the prostate approximately four weeks after seed implantation. The scan is used to evaluate the quality of the implant. Generally, an appointment with the urology department will be scheduled for the same day.
Assessing the treatment's success
After your treatment is complete, you will be monitored with serial PSA blood tests. Following radiation therapy, your PSA should fall but won't immediately reach its lowest value (nadir). It may take two to four years for your PSA to reach its nadir. Also keep in mind that your PSA may not decline steadily. In fact, you may see a temporary increase – called a bounce or spike – during the first 12 to 36 months after you complete radiation therapy. A bounce does not indicate treatment failure.
There is much debate over the most accurate means of detecting treatment failure after a course of radiation therapy. Several years ago, medical professionals established a consensus definition to systematize how they evaluate treatment outcomes. This definition defines treatment failure as three consecutive increases in the PSA value after the nadir has been reached. A more recent definition involves a PSA level that rises to an absolute value of 2 above the nadir, which has shown a strong association with subsequent disease progression. Often, however, further treatment is initiated before PSA levels rise this much.
The testosterone-PSA relationship after HT and RT
With hormone therapy (HT), testosterone levels fall, with a corresponding drop in PSA. After stopping HT, testosterone levels usually take from several months to a few years to recover to pre-HT levels. Longer HT courses lead to longer testosterone recovery times.
Because RT may not destroy all prostate cells (some noncancerous prostate cells may survive), as testosterone levels rise, a small amount of PSA may be produced – even in the absence of cancer. If the cancer has been eradicated, PSA levels should stabilize after testosterone recovery. The lower the PSA value after testosterone recovery, the more likely it is that prostate cancer eradication has been achieved.
Additional treatment
Most patients don't need further treatment after radiation therapy. Whether you need more treatment will be determined by your PSA levels, Gleason score, prostate cancer stage, and whether you had your daily treatments as scheduled, particularly for EBRT.
Regular post-treatment PSA evaluation is important in monitoring as well as determining whether you need any additional treatment. Should your cancer recur, the treatment options will in part depend on your initial treatment and the location of the recurrence (in the prostate itself, in pelvic lymph nodes, or at a distance from the prostate, such as in the bones). A PET scan is usually done with increasing PSA to identify the site of recurrence. Possible recommendations include additional or alternative forms of radiation therapy, prostatectomy, cryotherapy, hormone therapy, or promising new treatments under evaluation in clinical trials. Your team of health care providers will discuss appropriate treatment options and their recommendations with you.
This article was written by UCSF medical experts Osama M. Mohamad, MD, PhD, and Anthony C. Wong, MD, PhD, and UCSF patient advocates Nathan Roundy and Stan Rosenfeld. It was last reviewed in June 2022.
This information is for educational purposes only and is not intended to replace the advice of your doctor or other health care provider. We encourage you to discuss any questions or concerns you have with your provider.