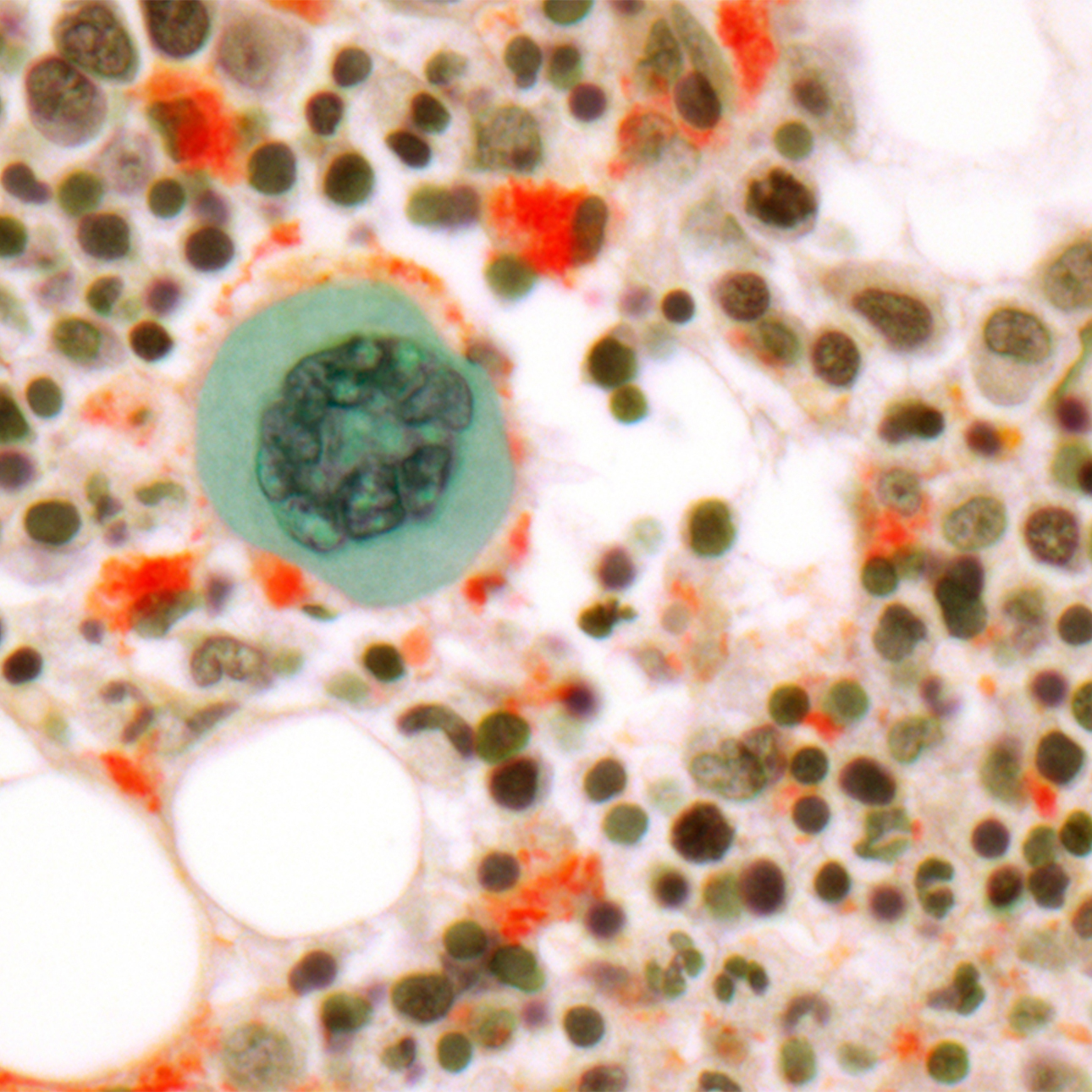
Chronic Lymphocytic Leukemia
Treatments
Most patients with early-stage chronic lymphocytic leukemia do not need any treatment when the disease is first diagnosed. These patients will live 10 to 15 years on average, and early treatment offers no advantage.
Treatment should start when the patient either has an advanced form of the disease or has an intermediate stage with significant symptoms, very enlarged lymph nodes or a rapid increase in the lymphocyte count (doubling in less than 12 months).
Chemotherapy
The most common treatment for CLL is the chemotherapy drug fludarabine. This is given intravenously (through an IV) five days a week once a month, for four to six months. Although the schedule for fludarabine is rather inconvenient, it causes only modest side effects, such as fatigue. Most patients will have a good response to this treatment and remain in remission without further treatment for two to three years.
The remission rate and length of remission are increased by adding a monoclonal antibody, Rituximab — also used to treat lymphoma — to fludarabine. The most aggressive regimen, fludarabine plus cyclophosphamide plus Rituximab (FCR), has the highest response rate (close to 100 percent), but at the expense of a high infection risk. A new chemotherapy drug, bendamustine, was recently approved for CLL; the patient receives two doses intravenously every month. Another agent, lenalidomide (Revlimid), which is FDA-approved for the treatment of multiple myeloma and certain types of myelodysplastic syndrome, has activity in CLL and is an oral medication.
Chlorambucil, an oral chemotherapy drug, may be used instead of fludarabine, especially for elderly or frail patients.
Stem Cell Transplantation
Allogeneic stem cell transplantation, also called bone and marrow transplantation (BMT), can be used to potentially cure CLL. However, this therapy is only used to treat the occasional young patient with aggressive CLL, since most patients with CLL live so long that the risk of transplant can seldom be justified.
Investigational Therapies
UCSF is dedicated to improving outcomes for CLL patients through the use of investigational therapies and clinical research trials. Clinical trials currently available to UCSF patients include Flavopiridol for CLL cases that don't respond to standard treatment. Flavopiridol kills CLL so quickly that blood salt and acid-base abnormalities arise, potentially causing kidney failure. The first cycle of Flavopiridol is given in the intensive care unit with kidney hemodialysis immediately available. Also available are national trials adding biologic agents, such as lenalidomide, to standard accepted CLL therapies.
Learn more about clinical trials and how they work.
UCSF Health medical specialists have reviewed this information. It is for educational purposes only and is not intended to replace the advice of your doctor or other health care provider. We encourage you to discuss any questions or concerns you may have with your provider.